Case Study: Web Strategy & Implementation
Clinical Diagnostics
I love turning complex challenges into clear, impactful solutions. For Bio-Rad’s Clinical Diagnostics redesign, I led a global website overhaul, aligning five divisions and 20+ stakeholders to create a user-focused, regulatory-compliant platform that drove a 1,230% surge in page visits.
Increase Website Conversions/Performance
Create a Positive User Experience
Update Pages to New Style/Template
Goals
+ Engaged Visits
+ Form Fills (MQLs)
- Bounce Rates/404s
Metrics
Context
I led the transformation of Bio-Rad’s Clinical Diagnostics website, a complex global platform mired in legacy systems and partial redesigns. My challenge was to unify five subgroups—Clinical Immunology, Infectious Disease, Clinical Systems, Immunohematology, and Quality Controls—into a cohesive, user-centric experience while adhering to strict brand guidelines and FDA regulations. With only partial control over the broader Bio-Rad ecosystem, I collaborated with Outbound Marketing and Product Managers to prioritize product-level updates, creating a scalable foundation for future customer journeys. By focusing on sub-sections of each portfolio, I streamlined SKU audits and aligned stakeholders across regions. My vision was clear: build a compliant, engaging platform that empowered teams and delighted users, setting the stage for measurable growth.
Key Challenges and Insights:
EXTERNAL
Duplicate pages, 404 Regional Errors, and Outdated SKUs
Not only causes search engines to ignore site information and not trust the website, ranking us lower.
Knowledge/Ownership Gap
Addressing the knowledge and ownership gap on managing SKU availability and regional visibility via SAP.
Process/Workflow To Publish
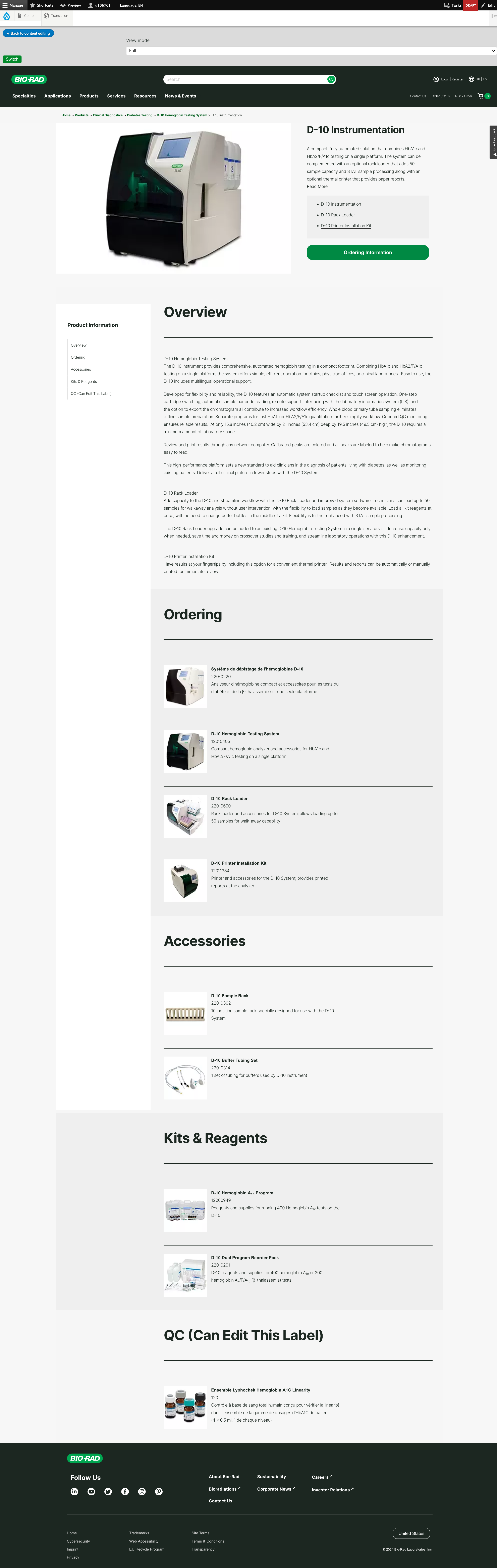
CDx (Clinical Diagnostics/Core Diagnostics) is divided up into divisions with stakeholders that manage distinct portfolios. Sometimes theses portfolios overlap. They are also regulated by the FDA and require approvals from Legal/Regulatory for launch.
INTERNAL
Stakeholders
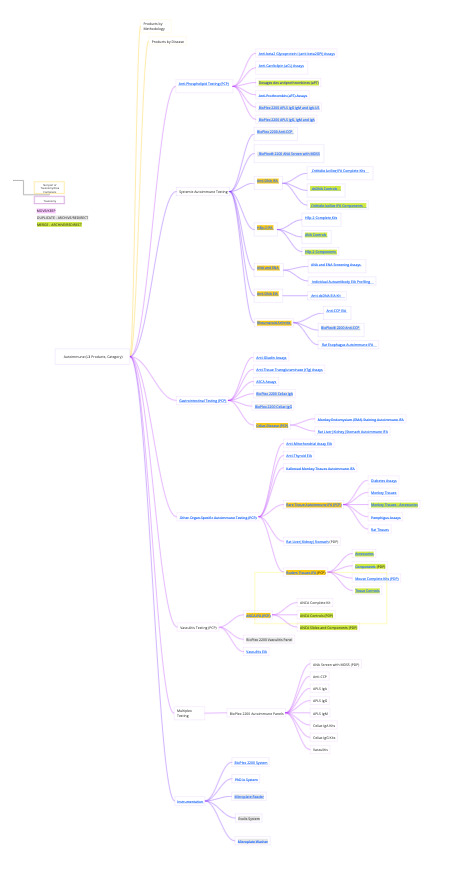
Different approaches and Stakeholder Opinions on organizing and sorting products and instruments on the website.
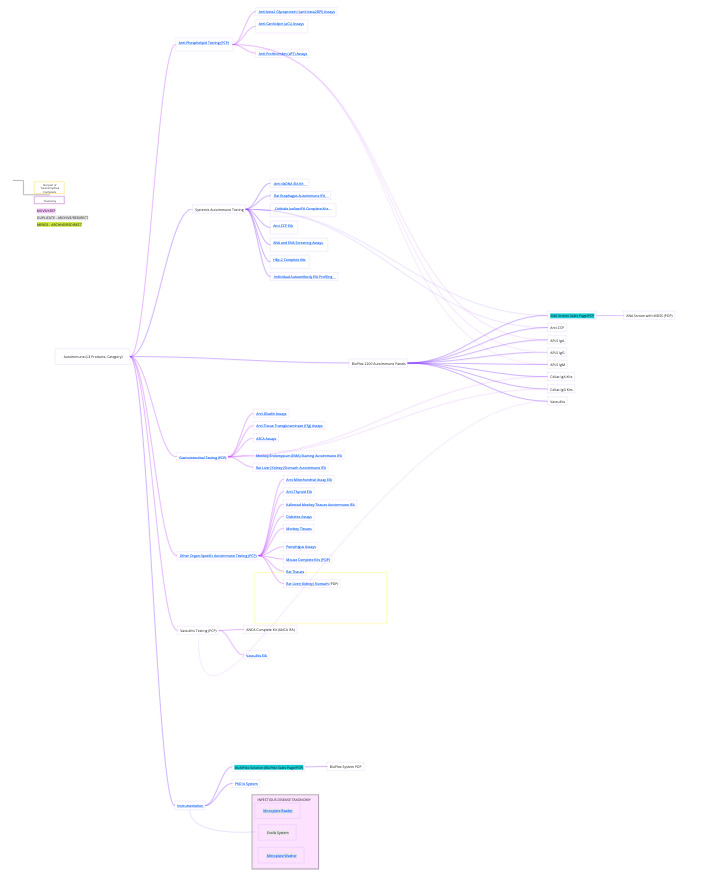
User Confusion with Site Architecture
How internals navigate the site vs how customers would search, duplicated pathways leading to duplicate pages per region (US, OUS, APAC)
Metrics/Tracking
Outdated forms and touchpoints are confusing to leads, but also hinder the ability for us to track page interactions. When forms are filled out, they are unable to be properly routed or attributed to a campaign.
Approach
Aligning Teams
Convened workshops with product and campaign managers to align on key pages, fostering a shared vision and prioritizing updates across each portfolio.
Collaborative Content Routing
Gained agreement on a routing process to streamline content flow through creative, copy, development, and regulatory teams, ensuring compliance and consistency while accelerating approvals.
Implementation and Publishing
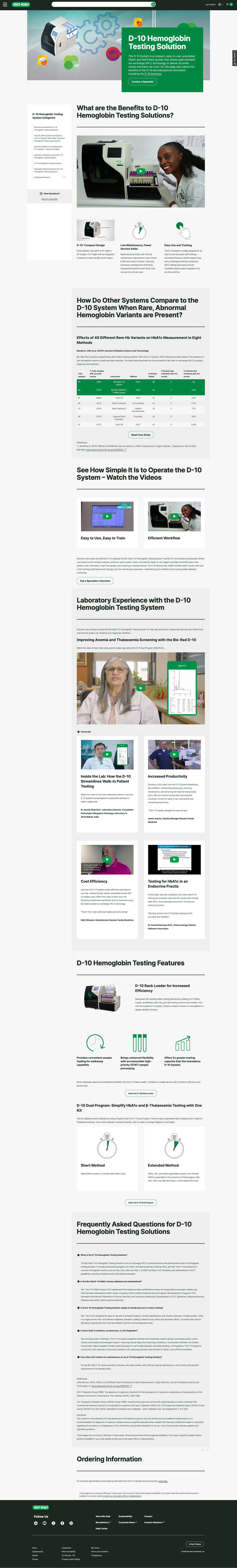
Audited the site to clean up 404s and duplicates, refined taxonomy for better user journeys, and oversaw publishing of 40+ multilingual pages, reducing deployment time by 25%.
Alignment
I teamed up with product portfolio and campaign managers to audit pages and map out the existing taxonomy, this enabled the teams to see the complex landscape we were working in, and all come to a clear objective on the goals and approach of the projects . My Redesign Resource Deck became the heartbeat of our workshops, guiding alignment and empowering teams with a go-to tool for future updates. This approach turned chaos and confusion into a shared vision, setting the stage for seamless execution.
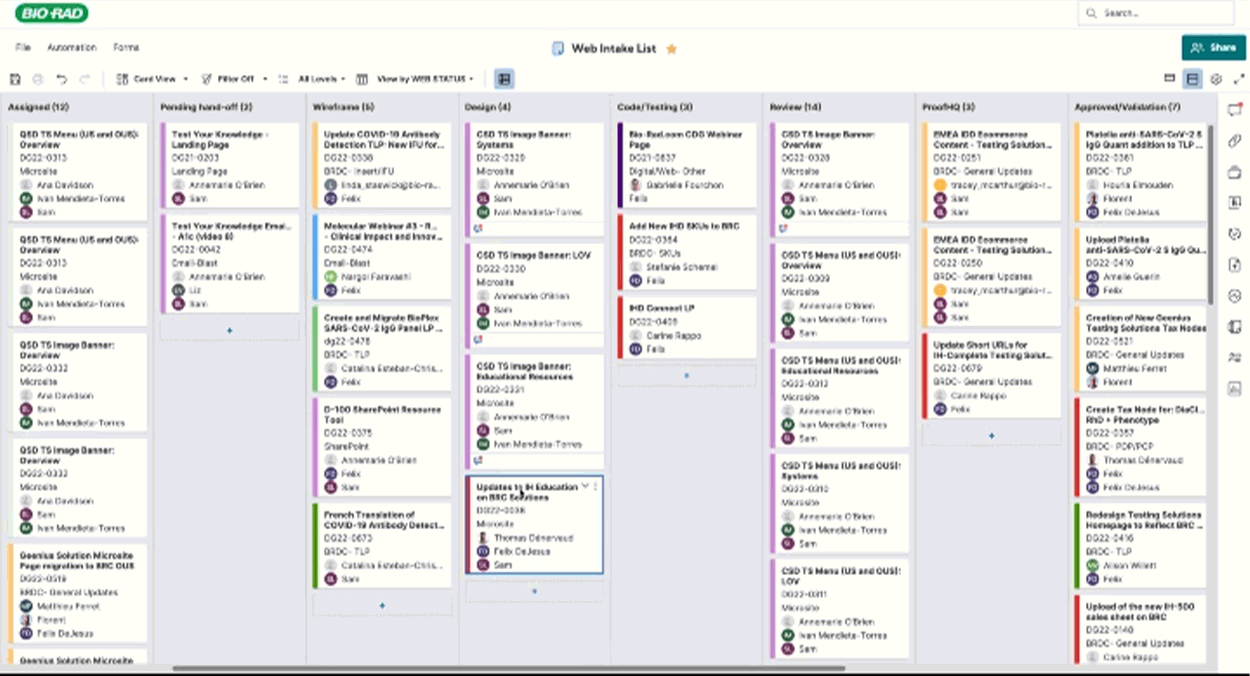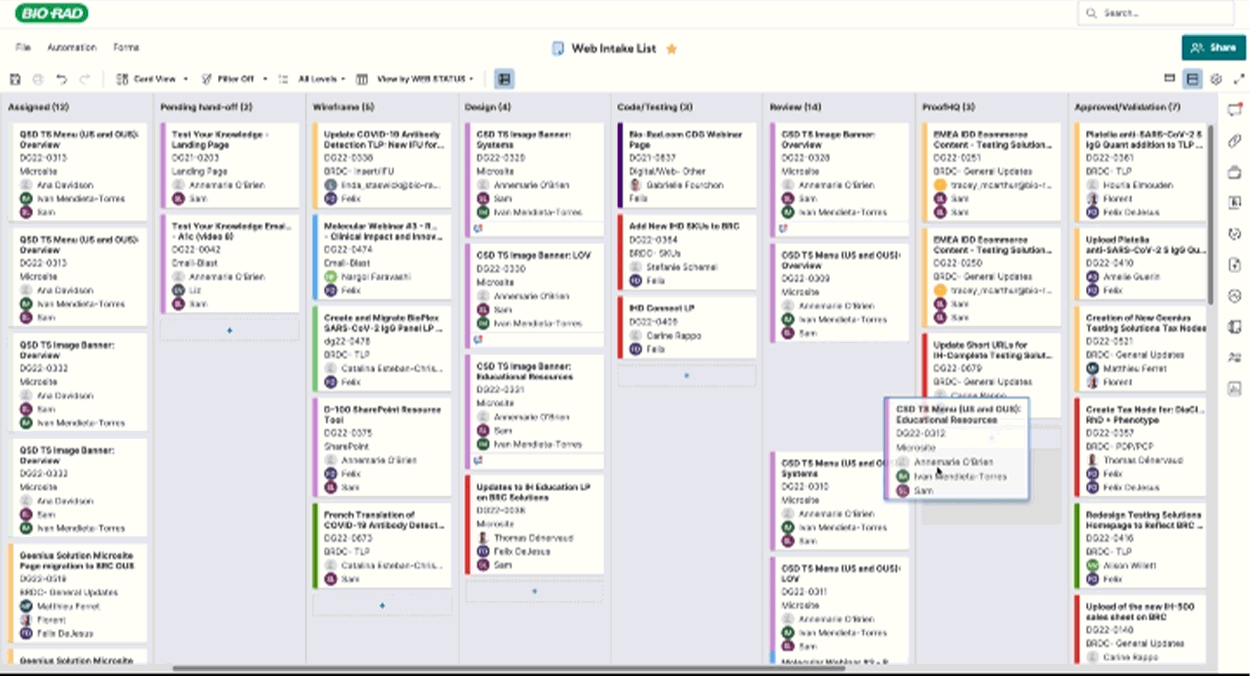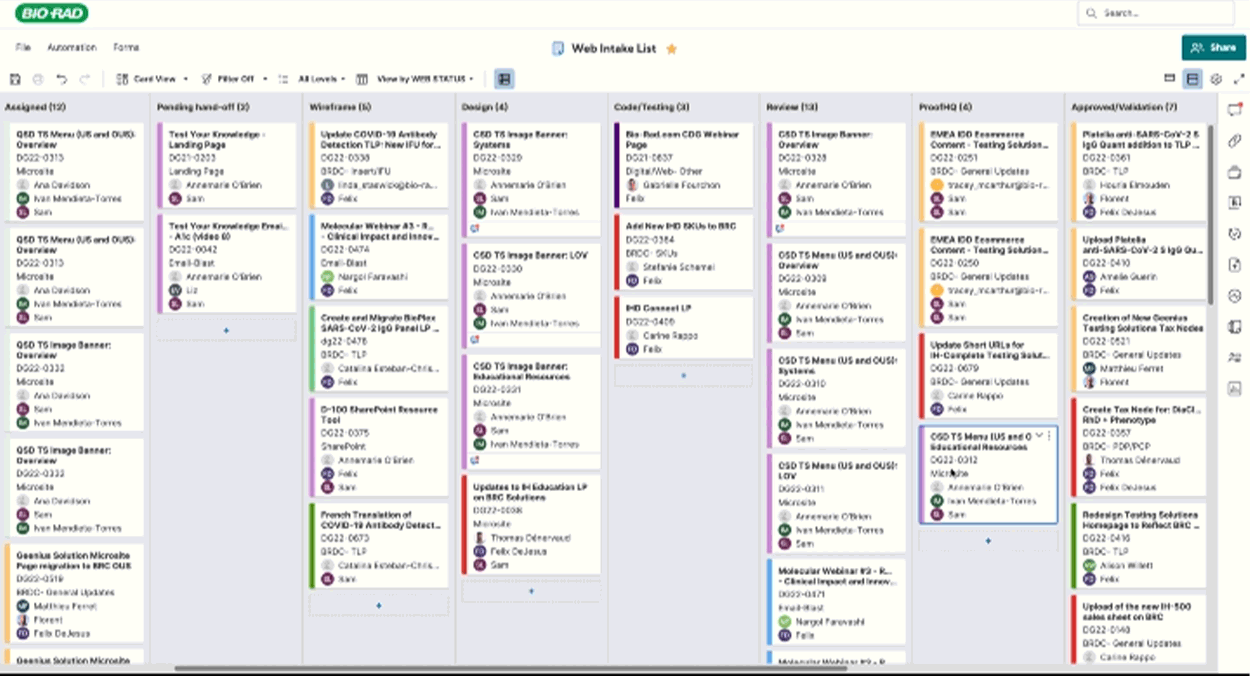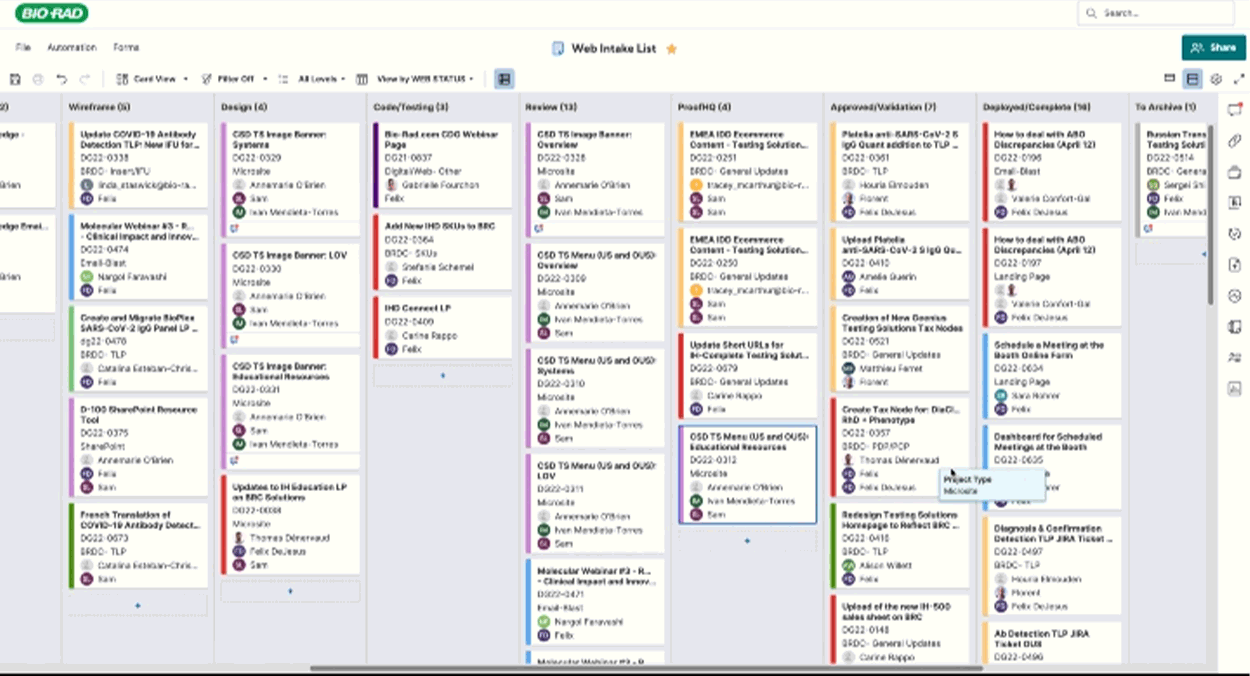
Routing & Project Management
I crafted project tracking dashboards and a SmartSheet E2E workflow to keep page updates on track, notifying the right teams at the right time. This system streamlined approvals and exported critical data—redirects, taxonomy tweaks, and new pages—empowering the web team to plan with precision and speed and quickly get resolution on issues flagged in the process.
Implementation & Publishing
As of 2025 Q2:
302 Pages Published, 422 Pages Archived, 211 Pages Translated across all 5 portfolios.
Results
OVERALL:
1,230%
Page Interactions (Clicks) Increase
100%
Page Visits Increase
57%
Reduction in duplicate pages, 404s, regional errors